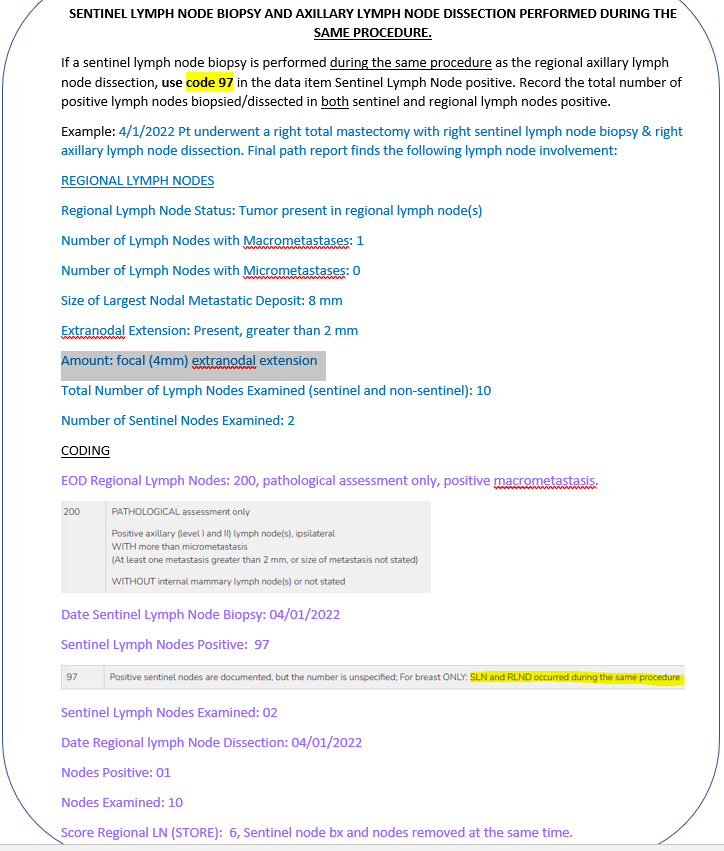
BREAST
Remember to text physical exam of breast and regional lymph node before first treatment to support clinical coding/findings!!!
Allred Score range (example, moderate-strong intensity score).
- Code SSDI Allred Score to X9 per https://cancerbulletin.facs.org/forums/forum/site-specific-data-items-grade-2018/129423-weak-to-stong#post129481
Ki-67 stated as range (example, 5-10%).
- Code SSDI to 5.1 in this example per https://cancerbulletin.facs.org/forums/forum/site-specific-data-items-grade-2018/120872-ki67
***************************
Polychythemia Vera Reportability
Clarification of polycythemia reportability--Is a diagnosis of “polycythemia NOS” reportable if a patient is treated with phlebotomy?
According to SEER, polycythemia (also known as polycythaemia or erythrocytosis) is a disease state in which the proportion of blood volume that is occupied by red blood cells increases. Blood volume proportions can be measured as hematocrit level. It can be due to an increase in the mass of red blood cells, "absolute polycythemia"; or to a decrease in the volume of plasma, "relative polycythemia".
The phlebotomy is a treatment for the excessive blood volume; therefore, a diagnosis of “polycythemia" without one of the modifying terms listed in the Heme DB under Alternative Names is NOT reportable.
(SINQ 20110060, last updated 6/29/12, source 2010 Heme & Lymph Manual & DB)
You may have heard April Fritz advise, during her fall workshop hematopoietic presentation, that if a patient diagnosed with polycythemia vera NOS is treated with phlebotomy, consider the polycythemia to be the reportable condition. However, KCR must follow SEER rules, as published in the SINQ answer.
[Per KCR via SEER SINQ; Published in October 2013 'In the Abstract']
Reportability--Heme & Lymphoid Neoplasms: Is polycythemia vera secondary to volume depletion reportable?
Answer: No, secondary polycythemia vera is not reportable. Primary polycythemia vera is a condition in which there is an overproduction of blood cells due to a neoplastic process. Secondary polycythemia vera is an over production of red blood cells caused by a co-morbidity, in this case, volume depletion.
(SINQ 20120049, last updated 7/17/12, source 2012 Heme & Lymph Manual & DB)
**************************
Melanoma
***************************
Prostate
When cancer is diagnosed incidentally (example, a cystoprostatectomy for bladder cancer) code EOD T to 800 (no evidence of primary tumor) in this field. If there is no documentation regarding a normal prostate evaluation (physical examination or imaging) prior to prostatectomy/autopsy, code 999 (unknown; extension not stated) in this field. https://staging.seer.cancer.gov/eod_public/input/2.1/prostate/eod_primary_tumor/?breadcrumbs=(~schema_list~),(~view_schema~,~prostate~)
***************************
Colon / Rectum
Copy and paste the entire Op Note to identify and support your primary site per priority order for coding primary site added below.
Review the pathology gross description for circumferential radial margin and remember to code CRM to millimeters, NOT centimeters.
RADIO-SENSITIZING CHEMOTHERAPY: If chemotherapy was provided as a radiosensitizer or radioprotectant DO NOT code as chemotherapy treatment. When chemotherapy is given for radiosensitization or radioprotection it is given in low doses that do not affect the cancer. Please review the treatment plan or reach out to the physicians administering treatment for confirmation.